Now that we’ve identified eligible patients for HARMS, we need to make an initial estimate of risk to guide how tightly we monitor this patient with urine drug testing (UDT). The key here is that there are a number of different strategies for risk stratification and physicians should take into account the time and energy available when deciding which method(s) to utilize. There is no “one size fits all” method…
Risk stratification plays a very important role in the HARMS Program as it guides how we prescribe and monitor opioid treatment. It is important to remember that this assessment of risk is an estimate based on the information available to us at one point in time, and that this risk estimate is continually evolving as new information arises. In fact, the whole point of UDT is to provide ongoing information to inform this risk estimate. UDT is therefore simply intended to inform risk/benefit balance, however it is not the only factor. This overall risk/benefit balance (including UDT results and other clinical factors) then informs our prescribing and monitoring of opioids (to see how we may adjust risk category as new information arises, see Chapter 9: Managing UDT Results).
Monitoring with UDT is informing the risk estimate, and the refined risk estimate feeds back to guide frequency of further UDT monitoring. Risk stratification and UDT therefore complement each other through each iteration of the cycle. We must remember that there are potential harms in doing UDT too frequently or too infrequently. More UDT means more inconvenience for the patient (and physician who addresses the results), increased costs to the healthcare system, and in the case of immunoassay UDT – higher risk of false positives or negatives and in the untrained interpreter risk of subsequent management errors. Likewise, less UDT may mean less information about the patient’s risk/benefit balance (risks in particular). Naturally, to balance the two variables of convenience and safety we estimate someone’s risk and conduct UDT at a rate concordant with that risk estimate. High risk patients get more frequent UDT in the interests of safety, at the expense of some convenience. Low risk patients get less frequent UDT in the interest of convenience, at the possible expense of safety. Each cycle of UDT (and subsequent risk adjustment as applicable) hones in on the best estimate of someone’s risk. Prescribing and monitoring strategies, meant to balance safety and convenience, are in essence adapting to the evolving risk estimate.
To summarize the importance of risk stratification, remember that the initial estimate guides where a patient starts on the spectrum of prescribing and monitoring, from tight to loose. But the iterative process of the HARMS Program is also important because it provides ongoing information to refine that risk estimate. We are trying to adapt to a moving target.
Now that the importance and clinical application of risk stratification has been described, let’s discuss different options to aid in risk stratification. Think of this list as a menu that you can pick and choose from. Do not feel compelled to use everything if you don’t have the capacity to do so. Remember, HARMS is meant to be practical and adaptable for your unique situation/setting. The highest yield indicator of risk is likely your clinical Gestalt.
Behavioural observations: There are numerous behavioural observations that have been reported to indicate an increased risk. See Appendix V for details of behaviours that indicate someone is at increased risk. Remember that while there are observations that may increase someone’s risk, an absence of these observations does not show that someone’s risk is zero, and that patients that have no behavioural concerns can still have concerns on urine drug testing.1,2 An absence of behavioural concerns is therefore not enough in itself to establish someone’s risk.
Medical History: There are numerous indicators of risk on a patient’s medical history. Many of these are variably captured using validated risk assessment tools (described further below)
| In terms of prescription opioid use disorder, a recent systematic review identified single high-quality studies which demonstrated the following risk factors as being associated with substantial increase in the likelihood of developing a prescription opioid use disorder3: |
|
| History of any pain disorder3 |
|
|
Other risk factors in the literature that may indicate someone is at increased risk for opioid misuse/opioid use disorder:
|
|
|
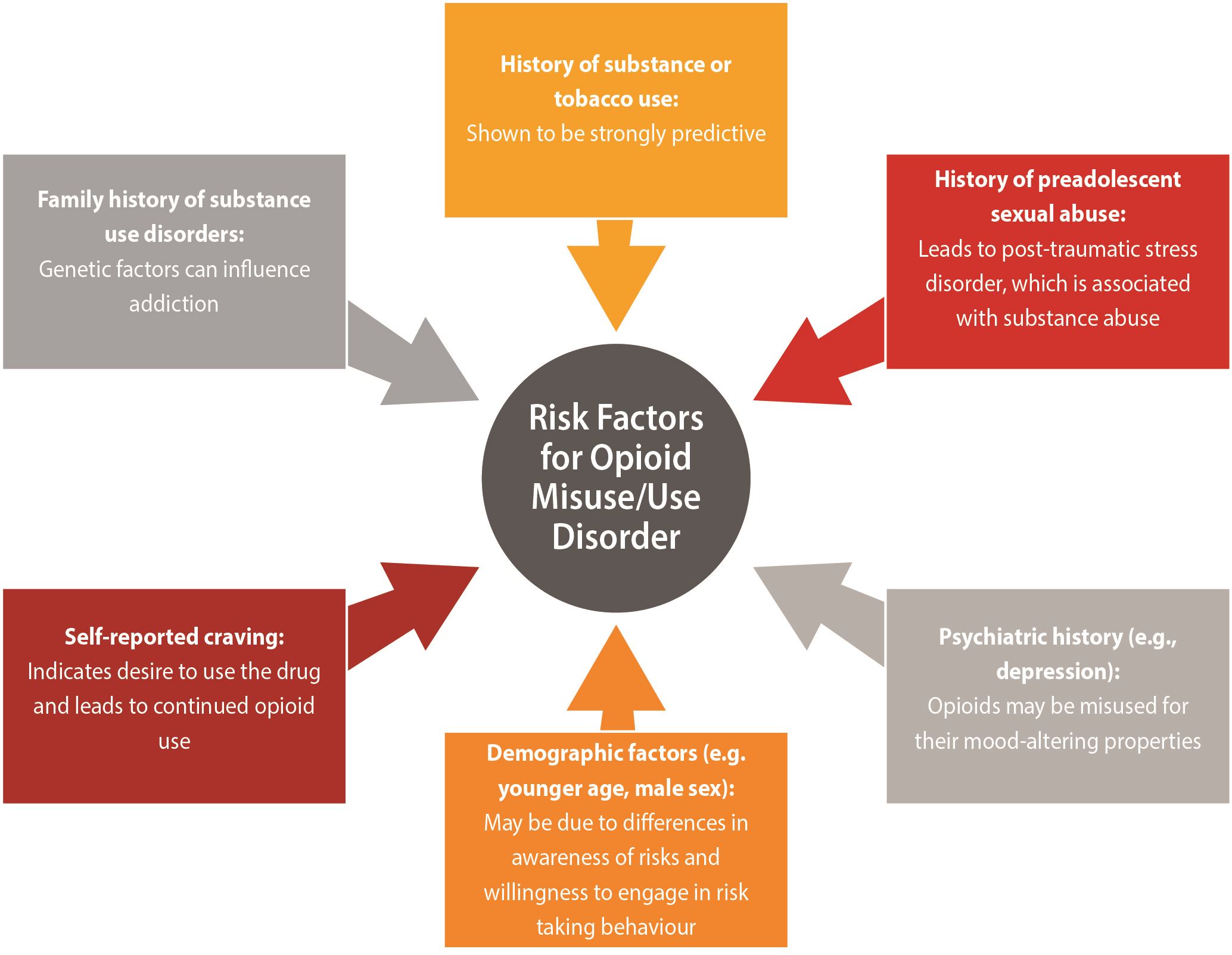
Reprinted with permission from Argoff et al. (2018).
Remember that this list is for opioid use disorder, but there are other potential harms of opioids including diversion and accidental overdose.
To elaborate further on the effect of higher doses, a study by Kaplovitch et al. found that patients escalated to high dose opioid therapy (defined as doses > 200mg of morphine or equivalent) were nearly 24 times as likely to die versus those patients who did not have escalated doses12. Risk of fatal opioid overdose has been shown with lower doses as well, with Canadian guidelines reporting risk as 0.1% for <20mg MED/day; 0.14% for 20-49mg MED/day; 0.18% for 50-99mg MED/day; and 0.23% for ≥ 100mg MED/day4. Not surprisingly, the risk of fatal overdose in patients with prior substance use disorder is even higher, with a 0.4% risk of fatal overdose at very low doses (<20 MED/day).4 This risk increases at higher doses.4
Although patients on higher doses of opioids are at higher risk of adverse effects, such as increased mortality, consensus is lacking on whether high dose cutoffs should contribute to risk stratification.12 Expert opinion considered using a high dose cutoff such as 120-mg morphine equivalent dose per day inadequate to identify high-risk patients alone as high doses may be a result of accommodating tolerance in some patients.6,13 Further, patients predisposed to opioid misuse could be at risk of misuse with even low to moderate doses.6
The HARMS Program leaves it open to individual preference whether to include the opioid daily dose in risk stratification. It was also felt that calculation of morphine milligram equivalents per day would be onerous for some clinicians and so this is not a requirement of the program. However, your clinic could choose to include this, especially if your clinic had a non-medical staff member perform the calculations. Previous recommendation thresholds for “watchful” doses have included 50/90/200 morphine equivalents per day.4
To calculate morphine equivalents, see opioid conversion tables in Appendix B-8 of the Canadian Guideline for Safe and Effective Use of Opioids for Chronic Non-Cancer Pain.4
There are several additional considerations, beyond the medical history described above, that may contribute to making more accurate estimates of risk at initial assessment.
Baseline UDT: Baseline UDT is recommended by several guidelines as it can indicate higher risk (it may indicate concern about a substance use disorder which, as described previously, puts someone at higher risk)
- Argoff et al. expert panel recommends definitive UDT (ie. LC-MS, GC-MS) at baseline for almost all patients with chronic pain being considered for an opioid trial as well as for ongoing monitoring of patients on opioids.6 For those continuing opioid therapy from another provider, UDT should be completed within three months of the first office visit6.
- The Canadian Guideline for Safe and Effective use of Opioids for Chronic Non-Cancer Pain (2017) states that clinicians may use baseline urine drug testing when considering patients for an opioid trial or for those who are currently on opioids. UDT can be repeated on an annual basis or more frequently for those at higher risk including those displaying aberrant behaviours.4 However an abstract report, which is listed in the guideline as the formal study of urine drug screening for risk mitigation, reported no difference in rates of opioid overdose for those who did or did not receive baseline urine drug testing.4 For those patients with chronic pain and a history of substance abuse, the Canadian guidelines recommend screening with a validated questionnaire (ie. CAGE for alcohol use, Current Opioid Misuse Measure (COMM) for opioid misuse) and suggest baseline UDT and periodically after.4
- The CDC Guideline for Prescribing Opioids for Chronic Pain (2016) found that there was limited evidence evaluating effectiveness of UDT for risk mitigation during opioid prescribing. Expert opinion does recommend that clinicians use UDT prior to initiating opioid therapy for chronic pain and periodically throughout therapy.14 There is a lack of consensus on whether this should apply to all patients and the frequency of monitoring thereafter. Most experts agreed annual testing at a minimum for all patients. Previous guidelines suggested more frequent testing for patients at higher risk for substance use disorder, however experts thought it was difficult to reliably identify patients at low risk with currently available tools.13,14
Now that we’ve covered some of the factors that might raise someone’s risk, let’s look at some of the validated tools that have attempted to put this together in estimating someone’s risk.
Risk Assessment Tools (Patient Questionnaires):
Some guidelines suggest utilizing risk assessment tools to assist the clinician in risk stratification and identifying risk of aberrant medication-taking behaviours.13,14 However, there is limited evidence supporting the use of currently available risk-stratification tools and when Klimas et al. tested performance by calculating likelihood ratios, most screening tools demonstrated poor diagnostic performance.3 The systematic review by Klimas et al. also found that commonly utilized risk assessment tools (Opioid Risk Tool, Brief Risk Questionnaire, Brief Risk Interview, and Screener and Opioid Assessment for Patients with Pain) were ineffective at discerning high from low risk patients.3 There is a need for further clinical validation of existing tools and for the development of more accurate tools that include additional risk factors.6
As a result, expert opinion emphasizes the importance of understanding why specific risk factors predict risk of aberrant drug use, and that risk stratification with a tool is only one component of a comprehensive assessment. Thus, clinicians are encouraged to choose a tool that matches their preference and work flow6. Some commonly used tools to assess risks with opioid use include: the Opioid Risk Tool (ORT), Screener and Opioid Assessment for Patients with Pain- Revised (SOAPP-R) tool, Current Opioid Misuse Measure (COMM) and the Diagnosis, Intractability, Risk, Efficacy (DIRE) tool. A summary of a few commonly used tools with diagnostic accuracies (e.g. sensitivity and specificity) can be seen below with a more comprehensive table found in Argoff et al. (DOI:10.1093/pm/pnx285)6. Again it should be emphasized that risk stratification is not static as personal circumstances can change and thus should be reassessed regularly6.
| Opioid Risk Tool (ORT) | Description | Time to complete | Diagnostic Accuracy | Validated | Additional Notes |
|---|---|---|---|---|---|
| Opioid Risk Tool (ORT) | Self-reported 10 item tool15 that assesses risk of aberrant drug-related behaviours16 | 1 minute13 | Sensitivity 20-99% and specificity 16-88% reported for detecting risk of opioid overdose, addiction, abuse or misuse using a cutoff score of > 4 or unspecified (5 studies)14 | Yes13 | |
| Screener and Opioid Assessment for Patients with Pain - Revised (SOAPP-R) | Self-reported 24 item tool13 that assesses risk of drug-related behaviours16 | < 10 minutes 13 | With a cutoff of >3 or unspecified - sensitivity 25-53% and specificity 62-73% for detecting risk of opioid overdose, addiction, abuse or misuse for likelihood ratios close to 1 (2 studies)14 | Yes13 | Designed to prevent patient deception15 Requires licensing agreement but no fee for individual clinical use13 |
| Screener and Opioid Assessment for Patients with Pain- 8 (SOAPP-8) | Self-reported 8 question tool that assesses risk for aberrant opioid-related behaviour17 | < SOAPP-R (< 10 mins) |
Sensitivity of 74% and a specificity of 66% | Yes17 | Adapted from the SOAPP-R to yield a shorter version while maximizing predictive accuracy17 |
| Current Opioid Misuse Measure (COMM) | Self-reported 17 item tool to identify patients receiving long-term opioid therapy who are exhibiting aberrant behaviours16 | < 10 minutes13 | With a cutoff score of ≥ 10: Sensitivity 74% and specificity 73%, and with a cutoff score of ≥ 9, sensitivity 77% and specificity 66% for the detection of aberrant drug-related behavior (1 study)15 | Yes13 | Requires licensing agreement but no fee for individual clinical use13 |
| Diagnosis, Intractability, Risk, Efficacy (DIRE) | Clinician interview 7 item tool to identify patients receiving long-term opioid therapy who are exhibiting aberrant behaviours18 | < 2 minutes13 | Sensitivity 94% and specificity 87% for poor vs good/fair adherence with a cutoff point of 13 (1 study)18 | Yes13 |
Table adapted from Argoff et al. (2018)
The HARMS Experience
There are a few practical elements that we have learned through experience when assigning someone’s risk. Physician time is crucial, and most of us don’t have time to manually apply validated risk stratification tools. At our clinic we tend to use clinical Gestalt (concerns about previous behaviour, medical history we are aware of with a focus on mental health and a history of addictions). Remember that this is an estimate! That estimate will be refined, so even if the estimate is off-the-mark, it can be refined over time as the patient declares himself/herself.
In a future version of the START-IT tool (see Chapter 7), we will be building optional, validated risk stratification tools into the program so that the information from these tools can be used without extra effort from the physician. Currently, the time to administer, compile and interpret the results from validated risk tools is too onerous for many physicians so this should overcome those obstacles.
In terms of how the risk estimates will be applied to real-life patients, the HARMS Program created various risk categories for patients prescribed opioids for CNCP (see Figure 3 – HARMS Risk Ladder): low, medium, high and structured (very high). The general theme is that the higher the risk, the more frequent the UDT and the shorter the medication dispensing interval (i.e. high risk means tighter control). Structured resembles a hybrid between a high-risk pain patient, and a patient being treated for opioid addiction. For an explanation of how a given risk level guides UDT frequency, and why these numbers were chosen, see Chapter 4.
Once patients are risk-stratified, the physician notifies the clinical administrator of the risk levels for each patient. When first starting the program, we simply took the list from the EMR query, and wrote “high”, “low” etc. beside any patient that would be part of the HARMS Program. As new patients are started on opioids or otherwise join the medical practice, we send a message to the clinical administrator for each patient saying “Please add to HARMS, [low/medium/high/structured] risk”. Alternatively, you may prefer to label them by their levels as demonstrated in the Risk Ladder and Chapter 4.
Cases

Case 1
55 year old male was found through the EMR query and you know that the patient has CNCP, has been on a stable daily dose of morphine (40mg/day) for a long time, has an "appropriate" diagnosis, is employed, and as far as you know his history is negative for red flags. How do you approach risk stratification?
This patient, by clinical Gestalt, appears to be low risk. In the interests of your own time, you do not apply risk stratification tools, or a baseline UDT prior to initial risk stratification. If you have the time or clinical capacity to do these extra steps then it is certainly reasonable, but in our program it is not necessary. You message the clinical administrator and ask to add to the HARMS Program as Low Risk (or level 1, from Risk Ladder).
Case 2
A 58 year old female has just moved to town. Her previous records indicate that she has been on oxycodone/acetaminophen 4 tabs/day for several years for chronic back pain. There is mention of alcohol use disorder but it seems to be in long-term remission. Would you consider any additional steps when assigning an initial risk category for this patient?
This patient presents a greater challenge because you don’t know her, and as a result your clinical Gestalt is “hazier”. This is someone who may warrant a more objective marker of risk (in addition to the usual history and physical). We would consider doing a baseline UDT on this patient as a relatively simple objective test. In fact, we would strongly consider performing a baseline UDT in all patients prescribed opioids for CNCP who are new to your practice, as this can help guide initial risk stratification. Once UDT results come back, if expected benefits outweigh risks then you can assign a formal risk category as she enters the HARMS Program.
Case 3
A 43 year old male is new to town and you are to be his family physician. You have his old records which indicate that he is on 10 tabs of oxycodone/acetaminophen per day with numerous early refill requests as well as dose escalations. He lacks a good diagnosis (he has no-showed to numerous appointments with pain specialists and for imaging). He has a history of alcohol use disorder, child abuse, and he was previously on a methadone program. How do you approach this patient?
This patient is particularly challenging as risks appear to outweigh benefits and you are likely not going to offer this patient long-term opioid treatment for pain. Ordering a baseline UDT may therefore not be helpful (See Chapter 9) if in your clinical judgement his risks outweigh benefits already and the UDT result would not change that. If you think that a UDT result may actually change your approach, then you may ask the patient to provide one. Consider that if UDT is unexpected this may make the ensuing conversation easier, however if UDT is expected then it may make the ensuing conversation more difficult if you are uncomfortable prescribing long-term anyway and will not offer patient this treatment option. The challenge in this scenario is not in deciding exactly what to do with his opioids, but instead how you do this. Critically important at this visit is compassion and open communication with the patient. The goal is to build/maintain a therapeutic relationship, as you adapt your treatment strategies to his suffering. See Appendix VI, VII, VIII. If you establish that he is indeed addicted to opioids and he acknowledges this, then offering him in a non-judgemental way help for this (with OAT) so that he doesn’t have to go through withdrawal may be enough to establish an initial therapeutic alliance.
Chapter Pearls

- Risk stratification is critical in guiding how systematic UDT (the HARMS program) is applied for a given patient. We want to prioritize safety while also acknowledging patient convenience. This means more frequent UDT for higher risk patients, and less frequent UDT for lower risk patients.
- Remember that our observations of behaviour are not sufficient to exclude people at risk, and patients can withhold information about illicit drug use.
- The initial risk stratification is an estimate based on information at one point in time. It will be refined over time in response to UDT results and other clinical observations.
- HARMS is meant to be practical - when it comes to risk stratification, use what you have time for. We don’t routinely use validated risk stratification tools in our practice. However this may change with the automated application of these tools coming in a future version of START-IT.
REFERENCES:
- McCarberg BH. A critical assessment of opioid treatment adherence using urine drug testing in chronic pain management. Postgrad Med. 2011;123(6):124-131. doi:10.3810/pgm.2011.11.2502
- Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97(4):1097-1102, table of contents.
- Klimas J, Gorfinkel L, Fairbairn N, et al. Strategies to Identify Patient Risks of Prescription Opioid Addiction When Initiating Opioids for Pain: A Systematic Review. JAMA Netw Open. 2019;2(5):e193365-e193365. doi:10.1001/jamanetworkopen.2019.3365
- Busse J. The 2017 Canadian Guideline for Opioids for Chronic Non-Cancer Pain. 2017.
- Staines GL, Magura S, Foote J, Deluca A, Kosanke N. Polysubstance use among alcoholics. J Addict Dis. 2001;20(4):53-69.
- Argoff CE, Alford DP, Fudin J, et al. Rational Urine Drug Monitoring in Patients Receiving Opioids for Chronic Pain: Consensus Recommendations. Pain Med Malden Mass. 2018;19(1):97-117. doi:10.1093/pm/pnx285
- Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
- Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med Malden Mass. 2005;6(6):432-442. doi:10.1111/j.1526-4637.2005.00072.x
- Substance abuse and Mental Health Services Administration. Results from the 2003 National Survey on Drug Use and Health: National Findings. https://rhyclearinghouse.acf.hhs.gov/library/2004/results-2003-national-survey-drug-use-and-health-national-findings. Published 2004. Accessed June 30, 2019.
- Prescott C. Sex Differences in the Genetic Risk for Alcoholism. https://pubs.niaaa.nih.gov/publications/arh26-4/264-273.htm. Accessed June 29, 2019.
- Tsuang MT, Lyons MJ, Meyer JM, et al. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55(11):967-972.
- Kaplovitch E, Gomes T, Camacho X, Dhalla IA, Mamdani MM, Juurlink DN. Sex Differences in Dose Escalation and Overdose Death during Chronic Opioid Therapy: A Population-Based Cohort Study. PloS One. 2015;10(8):e0134550. doi:10.1371/journal.pone.0134550
- Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. 2015. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 28, 2019.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep. 2016;65(1):1-49. doi:10.15585/mmwr.rr6501e1
- Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain Off J Am Pain Soc. 2009;10(2):131-146. doi:10.1016/j.jpain.2008.10.009
- Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain Off J Am Pain Soc. 2009;10(2):113-130. doi:10.1016/j.jpain.2008.10.008
- Black RA, McCaffrey SA, Villapiano AJ, Jamison RN, Butler SF. Development and Validation of an Eight-Item Brief Form of the SOAPP-R (SOAPP-8). Pain Med Malden Mass. 2018;19(10):1982-1987. doi:10.1093/pm/pnx194
- Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain Off J Am Pain Soc. 2006;7(9):671-681. doi:10.116/j.jpain.2006.03.001
Now that HARMS Program patients have been identified and risk stratified, we need a system to keep this organized and make sure that UDT is actually happening at a frequency corresponding to risk…
