While START-IT interprets immunoassay (IA) automatically, not all clinics will use START-IT. This chapter will not only cover how to interpret the two main types of UDT – IA and the “confirmatory” testing (LC-MS) – it will also compare and contrast these options through a highly pragmatic, clinical lens…
There are two main methods of UDT:
The immunoassay (IA) urine drug test, also sometimes called “point-of-care” testing or “presumptive testing”, may be conducted in the office or at the lab. It involves collecting a urine sample and then, on-site, dipping a kit with a number of different drug panels. Results are available within less than 10 minutes. While quick and fairly inexpensive (our kits cost $4.50 including shipping for a 5-panel test), there are drawbacks. First of all, IA only detects drugs for those panels used (i.e. it checks for fewer drugs than LC-MS). Some IA panels have significant cross-reactivity which potentially leads to false positives. Some panels have low sensitivity leading to false negatives. Of most clinical relevance, if a patient denies a result seen on an IA test (patient claims it’s a false positive or false negative), it is very difficult to act/discuss potential concerns because the test is limited and you cannot always be confident in the result. This is why it is important to know how to apply confirmatory testing and understand its role and limitations.
In contrast, the liquid chromatography-mass spectrometry (LC-MS) test, also sometimes called “confirmatory testing”, is sent away to the lab. LC-MS typically takes 1-2 weeks to process but the time is often worth the wait. It has much higher specificity (in theory, the test should be 100% specific), however there is always room for associated human error labelling or transcribing results. It is also generally more sensitive than IA so it has less false negatives.1 It can test for innumerable drugs and metabolites, including carfentanyl and “bath salts” (synthetic cathinones). The drawbacks are that in addition to it taking much longer for the results to come back, it is costly to the system. In Ontario, it is OHIP covered but the lab expense alone costs the healthcare system ~$40/test. For completeness sake, you may also hear about gas chromatography-mass spectrometry (GC-MS) for confirmatory testing. As far as the clinician is concerned, the same principles of LC-MS apply to GC-MS.
You can see that there are pros and cons to both types of testing. Recommendations in the literature about how to choose which method of UDT are variable. Argoff et al. interviewed a panel of experts who work with UDT and they came to the consensus that confirmatory testing should be used for baseline and future monitoring of addiction risk assessment instead of accepting the lower sensitivity and specificity of IA.2 Although confirmatory testing is more expensive, if the clinician is uncertain about the results of IA they should be ordering a follow-up confirmatory test anyway, making the initial IA UDT result nearly irrelevant. In fact, there is some evidence that setting up the clinic to have samples go directly to confirmatory testing can reduce turnover time and costs.3 The 2017 Canadian Guidelines for Opioid Use lean towards using IA as a first line screening modality because it gives immediate results and is inexpensive.4 When IA results are unexpected then confirmatory testing with GC/LC-MS should be ordered.
Practically speaking, when choosing which UDT method to use (IA and/or LC-MS), it is very difficult if not impossible to act on an IA result alone. You therefore really need some capacity to order LC-MS. The challenge is ordering it in patients in which it is potentially helpful but minimizing ordering in patients which it’s not helpful (due to costs to the system). START-IT offers an innovative solution to this problem because it can automatically discern the level of concern with the IA result (although of course it cannot factor in your clinical Gestalt) and make a strength of recommendation for confirmatory testing. For example, if the patient directly disagrees with the result, START-IT recognizes this discrepancy and strongly recommends confirmatory testing. If the result is expected or the patient agrees with an unexpected result, then START-IT advises that confirmatory testing “may be considered” but is not always necessary. Confirmatory testing has advantages even in these scenarios where the patient agrees with the result, because it checks for drugs in addition to those tested for with the IA panels.
| Characteristic | Immunoassay | GC/LC-MS |
|---|---|---|
| Speed | < 10 minutes | 1-2 weeks |
| False Positives | Many instances of cross-reactivity and false positives (i.e. poppy seeds for opioid panels, ranitidine for amphetamine panel, oxycodone for morphine panel, etc.) | Extremely unlikely to have false positives. However, we have had case reports of improper reporting by the lab |
| False Negatives | Can get false negatives due to tampering, insufficient quantity consumed or metabolic factors | Can detect drugs in the presence of tampering, can detect metabolites at lower levels and can differentiate between parent drugs and their multiple metabolites |
| Number of metabolites analyzed per sample | Limited to number of panels on device (usually 5), although could use multiple devices/kits | Can test for every metabolite from one sample |
| Characteristic | ~$5/test for the kit, + human resources | ~$40.00/test |
Let’s describe these two different types of UDT in a little more detail, with specific, common examples illustrating key pearls.
IMMUNOASSAY
Basic science: this paragraph is not directly relevant to clinical practice and we would advise that you don’t need to know this, however we are also aware that some of you are curious about the basic science so have included it here. IA strips involve chromatography, antibodies and an agent that produces a signal we can detect. In the case of point-of-care IA, the signal that is produced is simply the presence or absence of a coloured strip to let us know if a certain drug or metabolite was in the sample tested. The urine will carry a chemically labelled antibody, by process of chromatography, across the test strip. The chemically labelled antibody will reach an area on the test strip coated with drug-protein conjugates and the antibody will bind to these conjugates. In the absence of any drug the antibodies will bind the conjugates, precipitate the chemical, and the label will become a detectable coloured line on the test strip showing a negative result (the second line). In the presence of drug the antibody sites will be occupied and no detectable line will form showing a positive drug result (a single line for the control).
Clinician’s Overview:
Now that some of the basic science is out of the way, let’s discuss a few caveats for IA. First of all, there is variability depending on the manufacturer (so not all “morphine” panels are created equal). Secondly, patients may tamper with their urine to either hide a drug that’s not supposed to be there, or add a drug they’re not taking that is supposed to be there (see Appendix III: Anti-tampering Techniques).
The test will generally result as “positive” or “negative”, however it very rarely may result as “invalid” (we’ve had one invalid result in 5 years) in which case it should be repeated immediately. The sensitivity and specificity for a panel, even for the same manufacturer, will have a natural variability depending on the dose and time the drug was taken, as well as individual variations in pharmacokinetics (absorption, distribution, metabolism and excretion). There are enormous lists of agents that have been reported to cause false positives for the IA various panels. Instead of covering these individually, we recommend you consult Moeller et al, 20175 or US Pharm. 20166 or if using START-IT then it will give you the more common causes of false positives for your specific results.
Now let’s go through scenarios and pearls when interpreting the most commonly used panels:
Morphine/Opiate Panel: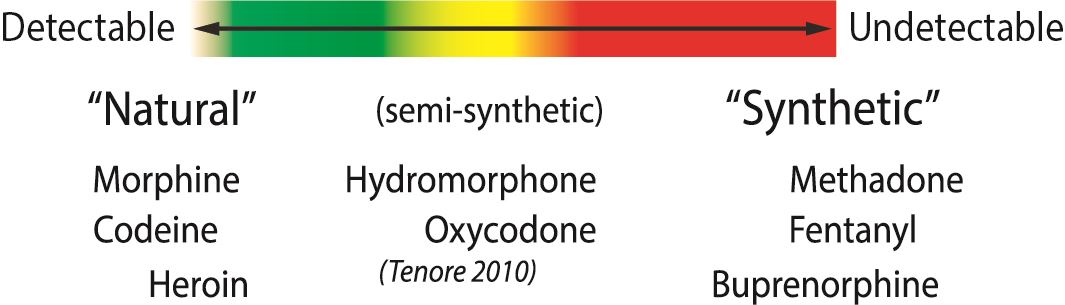
The morphine panel, also sometimes called the “opiate” panel (essentially the same thing), is very sensitive for detecting natural opioids. This includes codeine, morphine and (the mildly synthetic) heroin. The more synthetic an opioid, the less detectable. In fact, the fully synthetic opioids like fentanyl, methadone, tramadol and meperidine are not detectable on this panel. The semi-synthetics like oxycodone, hydromorphone and hydrocodone are variably detectable.
Practically, what this means is that if someone is prescribed hydromorphone for example, the morphine/opiate panel has limited utility. If the panel is positive, it suggests the person is taking hydromorphone (although it could be from another opioid). If the panel is negative, it doesn’t mean the person is not taking hydromorphone (high chance of false negative). If you are looking for a reliable test for the presence/ absence of the more synthetic opioids, then you should consider using specific panels for those drugs (they are commercially available for hydromorphone/hydrocodone, oxycodone, tramadol, methadone, fentanyl, and buprenorphine among others). Practically, you can start to go down the rabbit hole with number of panels so we use the 5 that we consider highest yield in our practice (Morphine, Oxycodone, EDDP (methadone), benzodiazepine and cocaine).
If the patient is prescribed morphine or codeine, then sensitivity of this panel is generally >90%. You will also note that this panel cannot distinguish between morphine/codeine/heroin. If relevant for your patient population, there is a specific IA panel for heroin which tests for a metabolite unique to heroin (6-MAM).
Oxycodone Panel:
The oxycodone panel unfortunately has a sensitivity that has been reported at 75%. This means that up to 25% of people who are actually taking their oxycodone may have a false negative. This of course is very important to know before accusing a patient of not taking his oxycodone just because the IA panel is negative.
Methadone (EDDP) Panel:
EDDP is the main metabolite of methadone. This is one of the most accurate IA panels with sensitivity of 96% and specificity of 99%.
Buprenorphine Panel:
Although studies have varied in the exact numbers, they all show the buprenorphine panel performs with high sensitivity (88-100%) and high specificity (87.5-100%). There have been reports of cross-reactivity with high concentrations of morphine, chloroquine and hydroxychloroquine.7-11
Benzodiazepine (BZD) Panel:
This is one of the more challenging panels because there is variability within the BZD class. In general, most BZD panels are designed to detect lipophilic BZD (oxazepam, temazepam, diazepam) and have a low sensitivity for clonazepam and lorazepam. However, even clonazepam and lorazepam are sometimes detected, so if a patient is prescribed lorazepam or clonazepam, this panel is generally not useful (a negative result cannot be acted on, a positive result supports that person took it but is not proof).
Cocaine Panel:
This is likely one of the most helpful panels when it’s positive. Specificity is reported as being as high as 100%12, so if cocaine IA panel is positive it means the person used cocaine. There are no agents known to cause false positives besides coca leaves. That said, sensitivity of this panel is low so it will frequently miss cocaine use.
Amphetamine Panel:
The amphetamine panel is the most difficult to interpret because of the many drugs that have cross-reactivities with this panel with many possibilities for false positives. Methamphetamine will likely be picked up by this panel but MDMA (ecstasy) is often missed and should be detected by an MDMA specific panel. Studies on the performance of amphetamine immunoassays vary greatly depending on the sample population they use because so many other drugs can cause false positives. In general this panel has been shown to have high sensitivities (around 100%) while the specificity can vary greatly (58-99%) depending on the patient population.13-16
Fentanyl Panel:
The fentanyl panel may be of increasing importance as more of it is found mixed into street drugs and for monitoring out-patients using fentanyl patches. There are fewer studies demonstrating the performance of fentanyl IA technologies but those that exist show high sensitivities (approx. 100%) and high specificities (86-99%). There has been report of cross reactivity with risperidone and trazodone. Fentanyl analogs like carfentanyl will also cause a cross-reaction but natural opioid analogues will not.17-19
Interpreting IA results:
To summarize, there is significant variability between the various IA panels in terms of sensitivity and specificity. Remember that results should be used to complement the rest of the clinical picture, and that with few exceptions IA can have false positives and negatives. First make sure that you know what the test result is suggesting, and if this suggestion is unexpected then consider explanations for a false positive/negative. Strongly consider confirmatory testing followed by an open discussion with the patient. It is very important that you understand the limitations of the test prior to discussing the results with the patient so as to not mismanage based on a misinterpretation or misunderstanding of the limitations of UDT. For more information about how to act on UDT (IA and/or LC-MS), see Chapter 9.
LIQUID CHROMATOGRAPHY-MASS SPECTROMETRY
Basic science: this paragraph is not directly relevant to clinical practice and we would advise that you don’t need to know this, however we are also aware that some of you are curious about the basic science so have included it here. Chromatography is the science of separating drugs, metabolites, proteins or other compounds based on their chemical properties. In chromatography you will always have a column with a stationary phase that is designed to retain these compounds in the column and a mobile phase that pushes the compounds out of the column. Based on chemical properties, every different compound will travel through the column at a different speed. In gas chromatography (GC) the stationary phase is a polymer and the mobile phase is a gas heated to extreme temperatures. The drugs or metabolites of interest must be also turned into a gas through extreme heat to be analyzed through gas chromatography. If it is not possible to become a gas the drug or metabolite will need to undertake a chemical derivatization reaction before entering the column. This increases the work to analyze your sample and limits the amount of substances that are possible to analyze. In liquid chromatography (LC) the sample is also placed on a column packed with a polymer but the mobile phase is pumped in at high pressures as a liquid solution. It is possible to analyze over 300 different substances at once using LC. The drugs or metabolites will be detected by a UV detector at the same time as their control would and can be accurately confirmed by tandem mass spectroscopy (MS) to identify the exact molecular weight of the substance leaving the column.20
LC-MS Clinician’s Overview:
Confirmatory testing is essential when the patient disagrees with the result suggested by presumptive testing (IA), or when you want to check for more drugs than are readily available on your IA panels. The LC-MS results include specific drugs and metabolites in the urine, and in theory should have a specificity of 100%. If a drug is detected, then the patient took it (or a parent drug). However, that said, we have had several examples of human error where a LC-MS report came back showing a drug was taken, but after adamant refusal by the patient and reinforcement from the rest of the clinical picture, the lab was contacted and there was a reporting error (i.e. the person didn’t actually have those drugs in their urine even though the report indicated that he did). The other time where you may get tricked, although not relevant in countries such as Canada where Vick’s Nasal inhaler is not available, is that Vick’s Nasal inhaler has methamphetamine, however it’s the enantiomer of the methamphetamine used in “speed” or crystal meth. The commonly used LC-MS cannot tell the difference. In other words, it might look like the person consumed “speed” when in fact it’s simply an over-the-counter nasal inhaler.
One of the more challenging aspects of interpreting LC-MS results is in knowing the numerous breakdown products. For example, codeine is metabolized into numerous other opioids and if you were not aware of this, you may see hydromorphone in someone’s urine who is prescribed codeine and falsely assume that he has been taking a non-prescribed opioid.
There are two ways to approach the vast amount of information when interpreting what a LC-MS result means. You could either start with the parent drug that the patient is supposed to be taking and look for the expected metabolite(s) to confirm consumption, or alternatively you could look at metabolites and work backwards to discern what this suggests about patient consumption. In general, it is easiest when looking at a prescribed drug to check for one of more metabolites. And when metabolite(s) show up that are not something you are expecting, you work backwards to determine what substance could cause this result.
One challenge that came up for us with several patients at our clinic revolved around impurities in prescribed medication. In an ideal world, a patient’s “morphine” medicine has 100% pure morphine, however unfortunately that’s not the case. As you can see from the table below, numerous opioids have impurities of closely related opioids. So if someone is on a high dose of one of these opioids, that could cause a “false positive” for another opioid on LC-MS.
| Commercial Active Pharmaceutical Ingredient (API) |
Process impurities |
Allowable Limit (%) |
Typical Observed (%) |
|---|---|---|---|
| Morphine | Codeine | 0.5 | 0.01 - 0.05 |
| Codeine | Morphine | 0.15 | 0.01 - 0.1 |
| Hydrocodone | Codeine | 0.15 | 0 - 0.1 |
| Hydromorphone | Morphine Hydrocodone |
0.15 0.1 |
0.0 - 0.025 0.0 - 0.025 |
| Oxycodone | Hydrocodone | 1 | 0.02 - 0.12 |
Table adapted from Shults, T. MRO Advisory: Critical Pre-Publication Information for MROs on Opiate Interpretations.21
Pearls for the more common LC-MS scenarios in clinical practice are described below:
Opiates (“natural” opioids like codeine/morphine and the mildly synthetic heroin):
Codeine is one of the most important opioids to know because it is the most commonly prescribed short-acting opioid in this province22 and it has so many metabolites that errors in interpretation are easy to make. As you can see below, codeine is broken down into morphine, hydromorphone and hydrocodone – all of whom are prescribable opioids (parent drugs) themselves. So if a patient has taken codeine, you cannot readily determine if he also ingested one of these other drugs. That said, if you see 6-MAM then this means that the person consumed heroin (it’s not part of codeine pathway). Another challenge is that poppy seeds actually contain very small amounts of codeine and morphine.23,24
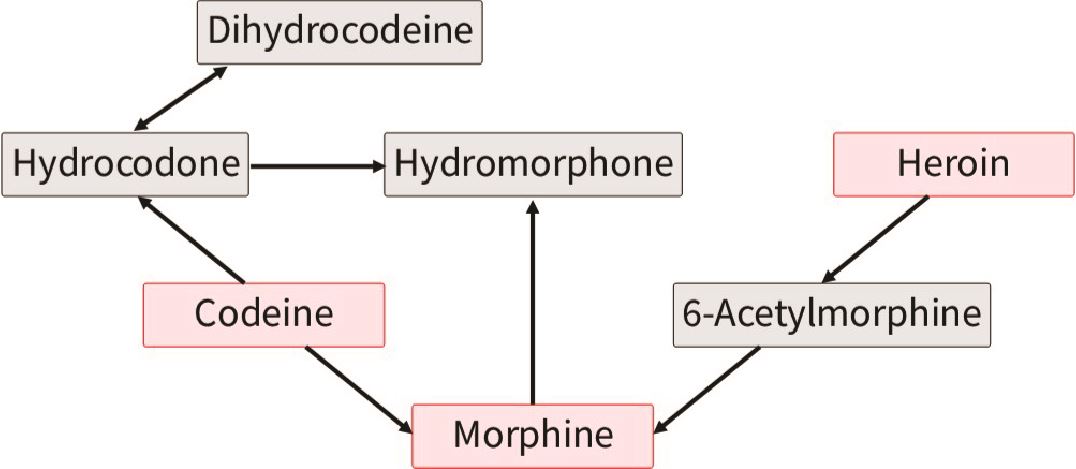
Figured adapted from Pesce et al. (2012)25
Likewise, you can see that if someone is taking morphine, then he may also have hydromorphone detected in his urine. While based on metabolism alone you would not expect someone prescribed hydromorphone to have morphine in his urine, or someone prescribed morphine to have codeine, remember from above that there can be impurities in the drug taken that are detectable (particularly at higher doses) and these do not necessarily indicate taking non-prescribed opioids.
Synthetic Opioids:
Contrasted to the opiates (“natural” opioids), the more synthetic opioids have their own metabolic pathways and are generally much simpler to interpret on LC-MS. Below are some examples of semi-synthetic and synthetic opioids, and what you may see on LC-MS:
- Oxycodone: Oxycodone, Oxymorphone, Noroxycodone
- Methadone: EDDP, Methadone
- Buprenorphine: Buprenorphine, Norbuprenorphine
- Tramadol: O-desmethyltramadol, N-Desmethyltramadol, tramadol
- Fentanyl: Norfentanyl, Fentanyl
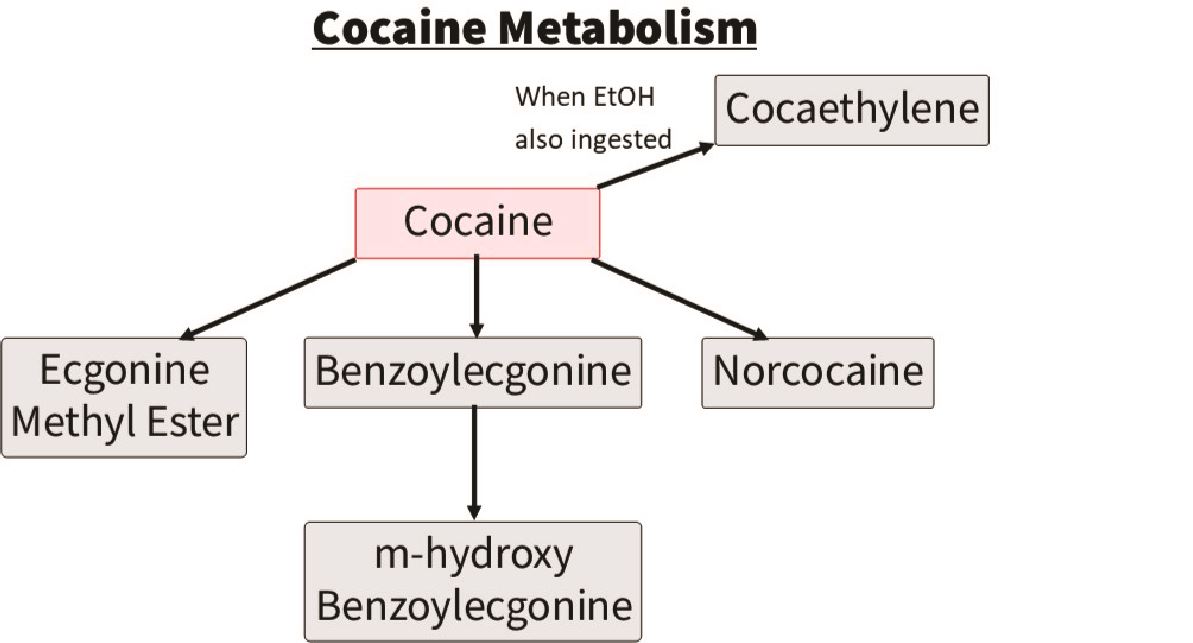 Figured adapted from Smolinska-Kempisty, et al. (2017)26
Figured adapted from Smolinska-Kempisty, et al. (2017)26
Cocaine:
The parent drug cocaine is rarely detected. The drug that is typically detected to indicate cocaine use is a metabolite of cocaine called benzoylecgonine. This stays in the urine ~3 days, however in heavy users can stay up to 10 days. The other drug commonly detected on LC-MS with cocaine use is levamisole, which is a cutting agent. You may also see cocaethylene which indicates alcohol and cocaine co-ingestion.
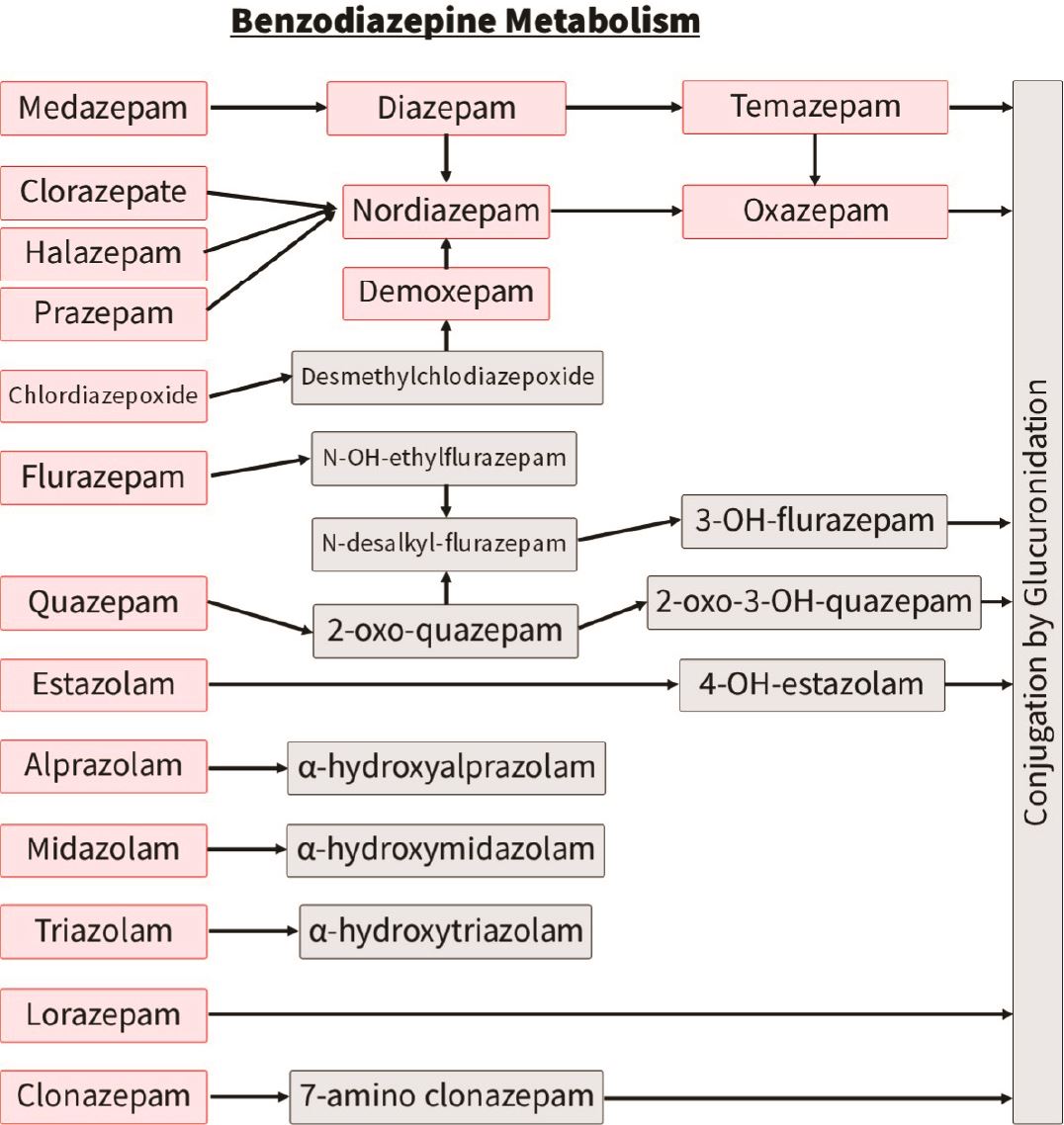 Figured adapted from Pesce et al. (2012)25
Figured adapted from Pesce et al. (2012)25
Benzodiazepines:
The most challenging benzodiazepines to interpret are, similar to opiates, those with numerous metabolites that themselves can be parent drugs. For example, temazepam and oxazepam in the adjacent chart.
Contrasted to diazepam and its metabolites, clonazepam and lorazepam have their own breakdown pathway and so these tend to be simpler to interpret on LC-MS
Miscellaneous:
A common scenario, even after doing this for many years, is that a metabolite shows up on LC-MS that you have never seen before and doesn’t seem to resemble any drug you’re familiar with. See case 4b below for an example of how you might navigate through this scenario.
FALSE NEGATIVES
False negatives can occur on both IA and LC-MS. There are several explanations for false negatives: 5,27
- Dilute urine (excess fluid intake, diuretic use, pediatric sample)
- Infrequent drug use
- Prolonged time since last use (detection windows vary greatly between drugs, and significant variability even within the same drug)
- Recent ingestion
- Insufficient quantity ingested
- Metabolic factors
- Inappropriate test used
- Elevated urine lactate
- Tampering

For quick UDT interpretation from your smartphone, we would highly recommend our free app “UrInterpret”. In addition to there being sections describing both HARMS and START-IT, there is a section with Cases where you can practice with realistic, common scenarios. The section that we use the most ourselves is called “Quick UDT”. It is a user-friendly way of getting quick answers to your UDT interpretation questions, such as,
For IA,
- The morphine panel is positive, what drugs may have caused this? Or conversely…
- My patient is prescribed hydromorphone, which panels would I expect to be positive?
For LC-MS,
- LC-MS detected hydromorphone, which drugs may have caused this? Or conversely…
- My patient is prescribed morphine, what metabolites may I see on LC-MS?
Visit our website www.harmsprogram.ca/urinterpret to see more about how it works.
Cases

Case 1
a. Mr Smith is prescribed long-acting codeine for chronic back pain at 100mg BID. He denies any other drugs. His IA panels come back positive for “morphine”, but negative for BZD, cocaine, EDDP (methadone) and oxycodone. Is this expected? Yes, codeine is metabolized into morphine and should be detected on morphine IA panel. False negatives are of course still possible and more likely with lower infrequent doses, so if it was negative it does not prove that he is not taking his codeine. In that case, await LC-MS results.
b. Your clinic sends his urine off for confirmatory testing and it comes back showing morphine, codeine, norcodeine, and norhydrocodone. Is this expected or unexpected? Expected - remember that codeine has numerous metabolites. If you’re not sure, look it up (our UDT app UrInterpret has a user-friendly way to quickly see metabolites for any drug).
Case 2
a. Mr. Green is prescribed long-acting oxycodone 40mg BID for chronic knee pain. He denies any other drugs. His IA panels come back positive for cocaine but negative for oxycodone, BZD and EDDP (methadone). i) How sure are you that he took cocaine? Very concerned, specificity is reported as being as high as 100%. How sure are you that he didn’t take his oxycodone? Not completely sure, remember that sensitivity of oxycodone panel is reported at 75% (although significant variability). However this patient is on a higher dose of oxycodone so false negative is less likely. Definitely need to send this result for confirmatory testing.
b. Confirmatory testing comes back showing benzoylecgonine, levamisole, cocaethylene, methamphetamines, amphetamines. i) Which metabolites here are related to cocaine? Benzoylecgonine is the main metabolite of cocaine, levamisole is a cutting agent used in cocaine, and cocaethylene is a metabolite of cocaine when consumed with alcohol. ii) How concerned are you now that patient is not taking prescribed oxycodone? Very concerned, absence of oxycodone in his urine - especially with a dose as high as 80mg daily - suggests he is not taking it. The presence of cocaine and methamphetamines, with the absence of the prescribed oxycodone - may suggest (although certainly not prove) that patient is selling/trading his oxycodone to support buying his drug(s) of choice.
Case 3
a. You inherit Mrs. Brown into your practice who is prescribed clonazepam 0.5mg BID as well as hydromorphone long-acting 6mg BID. You decide to do a baseline UDT for risk stratification and IA comes back positive on the “morphine” panel, but negative for BZD, cocaine, EDDP (methadone) and oxycodone. How concerned are you that patient is taking a non-prescribed opioid? Not at all concerned, hydromorphone as a semi-synthetic is variably detectable on the morphine panel and so the positive morphine is explained by the hydromorphone. Likewise, if the morphine panel was negative this would not suggest that patient isn’t taking it (this would likely be a false negative). How concerned are you that patient is not taking the prescribed clonazepam? Not at all concerned, sensitivity of BZD panel for clonazepam (and lorazepam) is low so false negatives are common.
b. LC-MS comes back positive for hydromorphone, 6-acetylmorphine (sometimes reported as 6-monoacetylmorphine or 6-MAM), clonazepam, amino-clonazepam. How concerned are you about the presence of 6-MAM? Very concerned, this indicates that patient consumed heroin and warrants further discussion with patient in a non-judgmental, supportive manner.
Case 4
You inherit Mrs. White who is prescribed diazepam 10mg BID and long-acting tramadol. Her IA result is positive for BZD but negative for morphine, oxycodone, EDDP (methadone) and cocaine. i) How concerned are you that she is not taking her tramadol? Not at all concerned - tramadol is a synthetic opioid and it is not detectable on the morphine panel. Would you have been concerned if her BZD panel was negative? Yes, although false negatives for BZD panel are possible, diazepam should be detected (contrasted to clonazepam and lorazepam which have high rates of false negatives).
a. LC-MS comes back showing nordiazepam, oxazepam, temazepam, tramadol, chlorpheniramine, pseudoephedrine, diphenhydramine, dextromethorphan and methcathinone. How concerned are you about the numerous benzodiazepines detected? Not at all concerned - remember that these are metabolites of diazepam. What do you do about the “methcathinone”? This is a real example to illustrate how to address some of the challenging scenarios. After years of applying UDT, we had never heard of “methcathinone”. A quick google search suggests it is a recreational drug that acts as a potent stimulant with euphoric effects. This seemed very unusual for the patient, so the lab clinical biochemist was contacted by email who confirmed that methcathinone can be seen as a “pharmaceutical impurity of some pseudoephedrine and ephedrine preparations”. In other words, OTC cough and cold medications can cause this. This is a good time to consider using our UrInterpret App If not sure about what a result means, or if it seems unusual for the patient, contact your clinical biochemist - they are very helpful. Try to have the discussion with the patient after you know what the result implies.
Case 5
a. Mr. Red is stratified as low-risk and stable long-term on four T#3 per day (120 dispensed q30d) for rheumatoid arthritis. He is called for random UDT and his IA comes back positive on the morphine and BZD panels. Cocaine, EDDP and Oxycodone panels are all negative. He denies using BZD. What do you do? Waiting for the confirmatory testing results is the first step. Result is definitely not so concerning that you need to act prior to LC-MS results coming back. If a BZD shows up on LC-MS then almost certainly the patient consumed one. Even if that was the case, you would still have to make sure that it wasn’t taken as part of a procedure that the patient forgot about (dental, surgical, ER, etc.). In real-life, this patient’s LC-MS came back showing no BZD. After review of potential false positives, what likely happened in this scenario was that the patient’s sertraline caused a false positive for the BZD panel.
Chapter Pearls

- Numerous pearls highlighted above for the common UDT results/scenarios.
- To order LC-MS, write “Broad spectrum urine tox screen” on the requisition. If you write “urine tox screen” then the lab often by default will process a “urine drug of abuse screen” which is a lab-based IA test, and you will therefore not have a confirmatory result.
- It is critical to interpret the UDT within the clinical context, and ideally you have confirmed the limitations (including potential false positives and negatives) prior to discussing with the patient. If you are not sure about what a result means, or if it seems surprising for a particular patient, then seek expert consultation (on LC-MS this often means consulting the lab clinical biochemist).
- Your clinic needs a method for sending the result for confirmatory testing. You can rarely act on the IA result alone. START-IT offers a helpful way of stratifying the level of concern with a result and guiding which samples to send for confirmatory testing.
REFERENCES:
- American Society of Addiction Medicine. Drug Testing: A White Paper of the American Society of Addiction Medicine (ASAM). 2013. https://www.asam.org/docs/default-source/public-policy-statements/drug-testing-a-white-paper-by-asam.pdf. Accessed May 30, 2019.
- Argoff CE, Alford DP, Fudin J, et al. Rational Urine Drug Monitoring in Patients Receiving Opioids for Chronic Pain: Consensus Recommendations. Pain Med Malden Mass. 2018;19(1):97-117. doi:10.1093/pm/pnx285
- Gencheva R, Petrides A, Kantartjis M, Tanasijevic M, Dahlin JL, Melanson S. Clinical Benefits of Direct-to-Definitive Testing for Monitoring Compliance in Pain Management. Pain Physician. 2018;21(6):E583-E592.
- Busse J. The 2017 Canadian Guideline for Opioids for Chronic Non-Cancer Pain. 2017.
- Moeller KE, Kissack JC, Atayee RS, Lee KC. Clinical Interpretation of Urine Drug Tests: What Clinicians Need to Know About Urine Drug Screens. Mayo Clin Proc. 2017;92(5):774-796. doi:10.1016/j.mayocp.2016.12.007
- Schwebach, A, Ball, J. Urine Drug Screening: Minimizing False-Positives and False-Negatives to Optimize Patient Care. https://www.uspharmacist.com/article/urine-drug-screening-minimizing-false-positives-and-false-negatives-to-optimize-patient-care. Accessed November 3, 2019.
- Berg J, Schjøtt JD, Fossan KO, Riedel B. Cross-reactivity of the CEDIA buprenorphine assay in drugs-of-abuse screening: influence of dose and metabolites of opioids. Subst Abuse Rehabil. October 2015:131. doi:10.2147/SAR.S88935
- Alves MNR, Piccinotti A, Tameni S, Polettini A. Evaluation of Buprenorphine LUCIO Immunoassay versus GC-MS Using Urines from a Workplace Drug Testing Program. J Anal Toxicol. 2013;37(3):175-178. doi:10.1093/jat/bkt006
- Melanson SEF, Snyder ML, Jarolim P, Flood JG. A New Highly Specific Buprenorphine Immunoassay for Monitoring Buprenorphine Compliance and Abuse. J Anal Toxicol. 2012;36(3):201-206. doi:10.1093/jat/bks003
- Hull MJ, Bierer MF, Griggs DA, Long WH, Nixon AL, Flood JG. Urinary buprenorphine concentrations in patients treated with suboxone as determined by liquid chromatography-mass spectrometry and CEDIA immunoassay. J Anal Toxicol. 2008;32(7):516-521. doi:10.1093/jat/32.7.516
- Leino A, Loo B-M. Comparison of three commercial tests for buprenorphine screening in urine. Ann Clin Biochem. 2007;44(Pt 6):563-565. doi:10.1258/000456307782268129
- Manchikanti L, Malla Y, Wargo BW, Fellows B. Comparative evaluation of the accuracy of immunoassay with liquid chromatography tandem mass spectrometry (LC/MS/MS) of urine drug testing (UDT) opioids and illicit drugs in chronic pain patients. Pain Physician. 2011;14(2):175-187
- Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76. doi:10.4065/83.1.66
- Agius R, Nadulski T, Kahl H-G, Dufaux B. Comparison of LUCIO®-direct ELISA with CEDIA immunoassay for ‘zero tolerance’ drug screening in urine as required by the German re-licensing guidelines: Comparison of ELISA with CEDIA for “zero tolerance” drug screening in urine. Drug Test Anal. 2013;5(6):390-399. doi:10.1002/dta.1455
- Huang M-K, Dai Y-S, Lee C-H, Liu C, Tsay W-I, Li J-H. Performance characteristics of DRI, CEDIA, and REMEDi systems for preliminary tests of amphetamines and opiates in human urine. J Anal Toxicol. 2006;30(1):61-64. doi:10.1093/jat/30.1.61
- Peace MR, Tarnai LD, Poklis A. Performance evaluation of four on-site drug-testing devices for detection of drugs of abuse in urine. J Anal Toxicol. 2000;24(7):589-594. doi:10.1093/jat/24.7.589
- Wang B-T, Colby JM, Wu AHB, Lynch KL. Cross-Reactivity of Acetylfentanyl and Risperidone With a Fentanyl Immunoassay. J Anal Toxicol. 2014;38(9):672-675. doi:10.1093/jat/bku103
- Snyder ML, Jarolim P, Melanson SEF. A new automated urine fentanyl immunoassay: Technical performance and clinical utility for monitoring fentanyl compliance. Clin Chim Acta. 2011;412(11-12):946-951. doi:10.1016/j.cca.2011.01.029
- Wang G, Huynh K, Barhate R, et al. Development of a homogeneous immunoassay for the detection of fentanyl in urine. Forensic Sci Int. 2011;206(1-3):127-131. doi:10.1016/j.forsciint.2010.07.022
- Fitzgerald RL, Rivera JD, Herold DA. Broad spectrum drug identification directly from urine, using liquid chromatography-tandem mass spectrometry. Clin Chem. 1999;45(8 Pt 1):1224-1234.
- Shults T. MRO Advisory: Critical Pre-Publication Information for MROs on Opiate Interpretations. https://www.aamro.com/docs/news/18.pdf. Accessed August 12, 2019.
- Gomes T, Pasricha S, Martins D, Greaves S. Behind the Prescriptions: A Snapshot of Opioid Use across All Ontarians. Toronto: Ontario Drug Policy Research Network; 2017.
- Struempler RE. Excretion of codeine and morphine following ingestion of poppy seeds. J Anal Toxicol. 1987;11(3):97-99. doi:10.1093/jat/11.3.97
- Zebelman AM, Troyer BL, Randall GL, Batjer JD. Detection of morphine and codeine following consumption of poppy seeds. J Anal Toxicol. 1987;11(3):131-132. doi:10.1093/jat/11.3.131
- Pesce A, West C, Egan City K, Strickland J. Interpretation of urine drug testing in pain patients. Pain Med Malden Mass. 2012;13(7):868-885. doi:10.1111/j.1526-4637.2012.01350.x
- Smolinska-Kempisty K, Ahmad OS, Guerreiro A, Karim K, Piletska E, Piletsky S. New potentiometric sensor based on molecularly imprinted nanoparticles for cocaine detection. Biosens Bioelectron. 2017;96:49-54. doi:10.1016/j.bios.2017.04.034
- Standridge JB, Adams SM, Zotos AP. Urine drug screening: a valuable office procedure. Am Fam Physician. 2010;81(5):635-640
Now that we’ve covered how to interpret UDT results, let’s look at how we might apply those results to the management of real patients…
